When Good Methods Go Bad: The Ghost Peak Problem Haunting Your LC-MS
Water contamination causes 60-70% of unexplained ghost peaks in LC-MS.
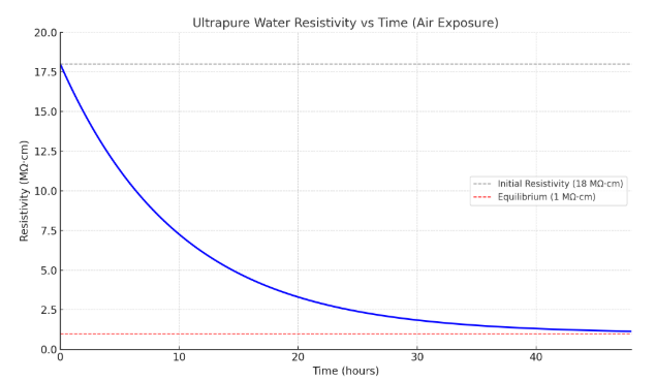
How Fast Ultrapure Water Goes Bad in a Lab
The 1-Minute to 48-Hour Problem
Byron Druss
The second you open ultrapure water, it starts to go downhill. Within 48 hours, water that began at 18 MΩ·cm (Type 1 standard) can drop to about 1 MΩ·cm. That’s a 94% purity loss.
Most people don’t realize it happens this fast. Many labs buy bottled ultrapure water, use it over days or weeks, and then wonder why their assays are inconsistent. The truth: once water meets air, it changes.
Why Stored Water Fails
Ultrapure water is “hungry.” It grabs carbon dioxide from the air the moment it’s exposed. That CO₂ turns into carbonic acid, which breaks down into ions. The ions ruin resistivity.
Here’s how fast it drops:
It still looks like water. But it’s not ultrapure anymore.
What That Means for Your Work
Bad water = bad data.
Storage: Open vs. Closed
Either way, you’re fighting a losing battle.
The Hidden Costs of Bottled Ultrapure Water
It’s not just a purity problem. It’s an efficiency problem.
Why Ultrapure Lab Water Systems Are Better
A point-of-use system makes ultrapure water when you need it. No storage. No guessing. No degradation.
Benefits:
For most labs, the system pays for itself in 12–18 months. Busy labs often break even even faster.
FAQs
How do I know if stored ultrapure water has gone bad?
Check it with a resistivity or conductivity meter. Below 10 MΩ·cm? Too compromised for sensitive work.
Which applications are most sensitive?
HPLC and mass spec are the worst hit. But cell culture and molecular biology take a big hit too. Even “simple” buffer prep can go wrong with degraded water.
Is switching to a system hard?
Not really. Install usually takes 1–2 days. Once it’s running, you’ll wonder why you ever bought bottles.
If I must store water, what’s best?
Use small sealed containers. Don’t keep longer than 48 hours if it’s for critical work. Nitrogen blanketing can stretch it, but it adds hassle and cost.
Bottom Line
Buying bottled ultrapure water feels easy. But it doesn’t stay pure once opened. It decays quickly and causes hidden costs—bad data, wasted time, and budget leaks.
An ultrapure lab water system solves all that. You get ultrapure water on demand, always fresh, always ready. Long term, it’s the smarter and cheaper choice.
And for those interested in the science:
The chart illustrates how the resistivity of ultrapure water drops from 18 MΩ·cm to around 1 MΩ·cm over 48 hours as it equilibrates with air. This decline is driven primarily by CO₂ absorption, which forms carbonic acid and releases ions, lowering the resistivity.
The chart is based on a simulated model, not direct experimental data. Here's the background:
Data Model Used
R(t)=17⋅e−kt+1R(t) = 17 \cdot e^{-kt} + 1
where:
Understanding water quality degradation helps labs make informed decisions about their ultrapure water strategy. The right approach depends on your specific applications, workflow requirements, and quality standards.
Want to learn more about the ins and outs of water treatment? Subscribe to this blog and we'll keep you posted.

Water contamination causes 60-70% of unexplained ghost peaks in LC-MS.

Your HPLC columns shouldn't fail after a few weeks. Under normal operating conditions, most reverse-phased C18 columns deliver stable performance for...

Ultrapure water (UPW) is like a lab's clean room, but in liquid form. At 18 MΩ-cm resistivity, Type I water isn't just "really clean"—it's so pure...

Your HPLC columns shouldn't fail after a few weeks. Under normal operating conditions, most reverse-phased C18 columns deliver stable performance for...
Ultrapure water systems are essential in labs performing high-precision work such as HPLC, molecular biology, and clinical diagnostics. These systems...

Water contamination causes 60-70% of unexplained ghost peaks in LC-MS.